


GMP stands for good manufacturing practice, a system that ensures products consistently adhere to quality standards. The Food and Drug Administration (FDA) put GMP regulations in place to minimize the risks involved in producing goods, such as supplements and other pharmaceuticals. GMP covers all aspects of production, from the materials, manufacturing location, and equipment to the training, PPE, and personal hygiene of staff. Being a certified GMP manufacturer is of the utmost importance. It is nearly impossible for a consumer to determine if a drug is safe or effective by simply looking at the pill. This is why GMPs are necessary, as they protect consumers from potentially harmful medicines and supplements. GMP certification not only gives manufacturers a strong reputation as trustworthy and responsible facilities, but it also shows that they produce their products at the highest quality possible.

In a highly competitive and demanding industry, ensuring sustainability and acquiring a competitive advantage is challenging for most businesses. ISO 9001 Quality Management System (QMS) is a globally recognized standard and has proven to be vastly beneficial for all types of organizations, regardless of their size. ISO 9001 helps organizations of all sizes and sectors to improve their performance, meet customer expectations, and demonstrate their commitment to quality. Its requirements define how to establish, implement, maintain, and continually improve a quality management system (QMS). Implementing ISO 9001 means our organization has put in place effective processes and trained staff to deliver flawless products or services time after time. Business benefits include: Customer confidence, Effective complaint resolution, Process improvement, and Ongoing optimization.

The scope of the HACCP certificate in our company includes two products, caps and spoons for baby milk powder, and shows the company's commitment to food safety.
HACCP is the most internationally recognized system based on safe food production from a preventative approach.

FSSC 22000 certifies the food, feed, and packaging safety systems of companies in the food chain that process or manufacture animal products, perishable vegetable products, products with a long shelf life, and other food ingredients. Certifying to FSSC 22000 addresses food safety and quality management in: Food and ingredient manufacturing Food packaging manufacturing Storage, distribution, transportation, and logistics. The scope of FSSC in our company is related to caps and spoons for baby milk powder. Compliance with the requirements of this globally recognized standard can help: Assure that food has been produced, prepared, and handled according to the most recognized standards(ISO 22000:2018/ISO TS 22002:4/Additional requirements)
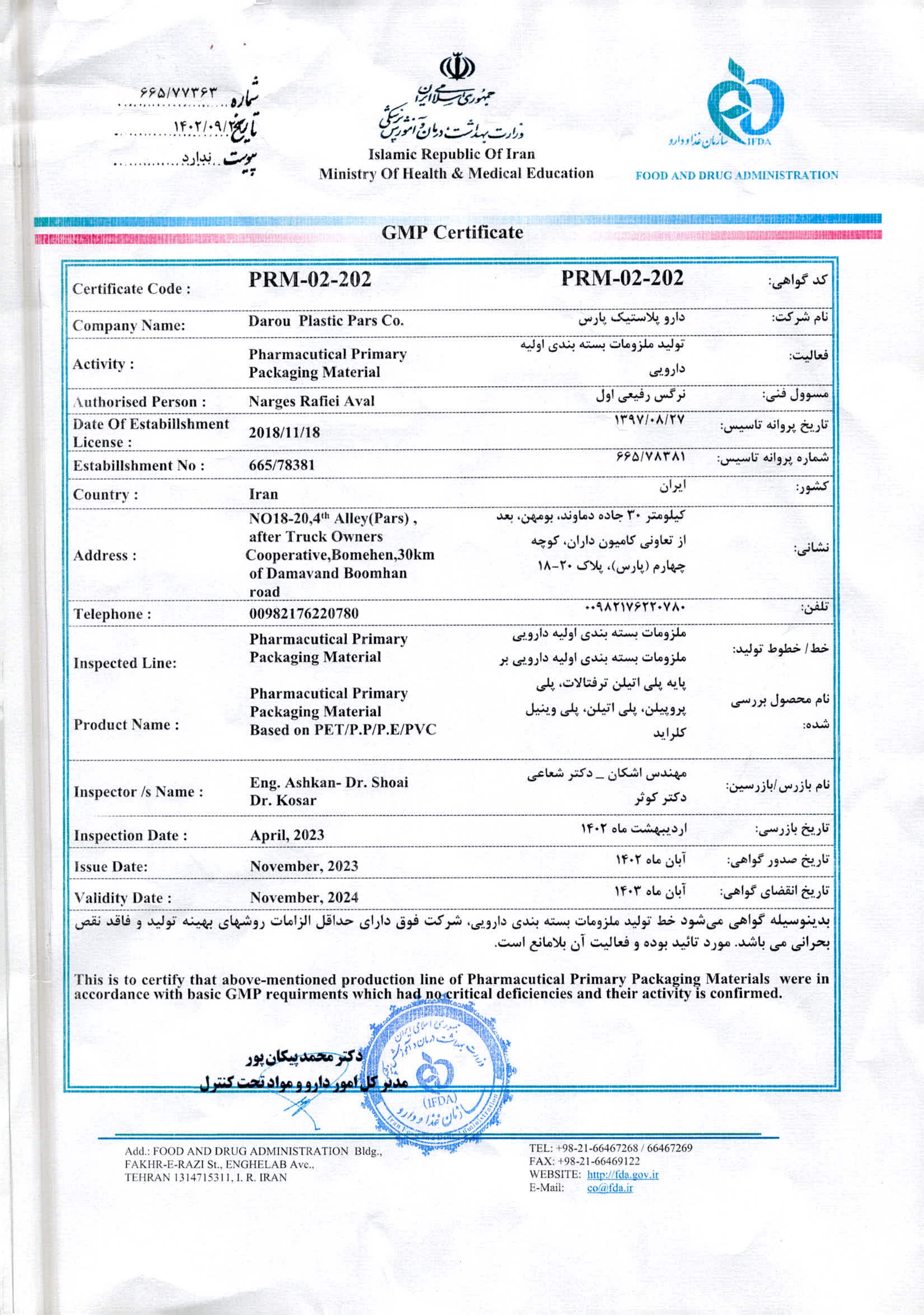
GMP stands for good manufacturing practice, a system that ensures products consistently adhere to quality standards. The Food and Drug Administration (FDA) put GMP regulations in place to minimize the risks involved in producing goods, such as supplements and other pharmaceuticals. GMP covers all aspects of production, from the materials, manufacturing location, and equipment to the training, PPE, and personal hygiene of staff. Being a certified GMP manufacturer is of the utmost importance. It is nearly impossible for a consumer to determine if a drug is safe or effective by simply looking at the pill. This is why GMPs are necessary, as they protect consumers from potentially harmful medicines and supplements. GMP certification not only gives manufacturers a strong reputation as trustworthy and responsible facilities, but it also shows that they produce their products at the highest quality possible.